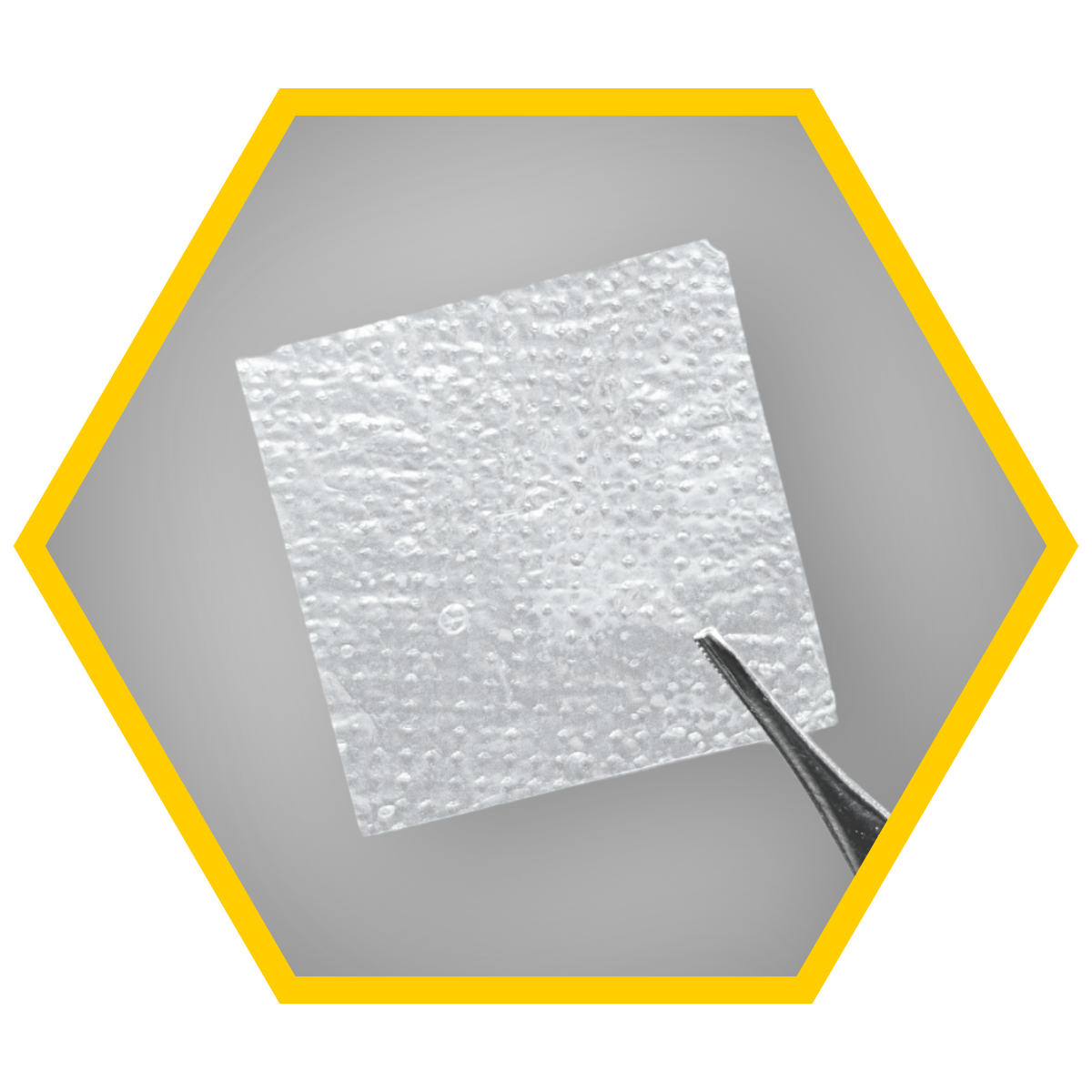
What is CELERA?
CELERA™ human amnion chorion membrane allografts are minimally manipulated and dehydrated. They are intended for use as a wound cover or skin substitute for cutaneous wounds. They support the healing cascade and protect the wound bed to aid in the development of granulation tissue in acute and chronic wounds.¹
Get started with CELERA today!
CELERA allografts provide a human biocompatible extracellular matrix (ECM)
CELERA allografts retain the structural and functional characteristics of native placental membranes, and they provide a human biocompatible extracellular matrix (ECM).
CELERA human placental allografts (361 HCT/Ps) are intended for use as a natural biologic wound covering or skin substitute for cutaneous wounds. CELERA allografts are intended for use by qualified healthcare professionals for application in a physician office, outpatient, or inpatient setting.
CELERA Product Benefits
Product Advantages
- Easy to apply
- Shelf-stable*
- 5-year shelf life
- Terminally sterilized for additional level of safety
- Compatible with compression therapy, Negative Pressure Wound Therapy (NPWT) and Hyperbaric Oxygen Therapy (HBOT)
*See Instructions for Use
Clinical Use Examples
- Acute Wounds
- Chronic Wounds
- Post-Debridement
- Dehisced Wounds
- Diabetic Foot Ulcers (DFUs)
- Venous Leg Ulcers (VLUs)
- Pressure Ulcers (PUs)
- Mohs Repair
- Deep or Tunneling Wounds
CELERA™ (Q4259)
Dehydrated Human Amniotic Membrane Allograft
| PART NUMBER | SIZE | SQ CM | UPC/GTIN |
| CEL-26222 | 2 cm x 2 cm sheet | 4 | 850046045240 |
| CEL-26223 | 2 cm x 3 cm sheet | 6 | 850046045257 |
| CEL-26224 | 2 cm x 4 cm sheet | 8 | 850046045264 |
| CEL-26244 | 4 cm x 4 cm sheet | 16 | 850046045295 |
| CEL-26246 | 4 cm x 6 cm sheet | 24 | 850046045301 |
| CEL-26248 | 4 cm x 8 cm sheet | 32 | 850046045318 |
REFERENCES: 1. Niknejad H, Peirovi H, Jorjani M, Ahmadiani A, Ghanavi J, Seifalian AM. Properties of the amniotic membrane for potential use in tissue engineering. Eur Cell Mater. 2008;15:88-99.