
Proven Solutions For Challenging Wounds
Acute and chronic wounds may need an advanced intervention to help rebalance the wound bed, facilitating normalization of the healing cascade. MIMEDX offers an array of advanced placental-based allografts that may be used in a variety of complex wound applications to provide valuable benefits in patient care.
Product Application & Experience Videos
Clinical Areas of Use
- Specialties include: podiatry, plastic
reconstructive, dermatology/ Mohs, vascular, general - Diabetic foot ulcers (DFUs)
- Venous leg ulcers (VLUs)
- Pressure ulcers
- Post-debridement
- Complex defects
- Limb salvage
- Mohs closure
Product Advantages
- Provides a protective barrier & human biocompatible extracellular matrix (ECM)
- Protects the wound bed to aid in the development of granulation tissue in acute & chronic wounds
- Contains native regulatory proteins
- Supports the healing cascade
- Available in a variety of sizes and configurations
- Backed by validated Level I evidence
Case Study Examples
Acute and chronic wounds may benefit from advanced interventions with the goal of rebalancing the wound bed, facilitating normalization of the healing cascade. Discover how clinicians used EPIFIX and EPICORD across a variety of wound types and applications.
Case Example 1
Diabetic Foot Ulcer (DFU): EPIFIX + NPWT
EPIFIX in a neuropathic diabetic patient with scheduled amputation avoided.
Comorbidities: DM and neuropathy
Goal: Secondary Intention Wound Closure
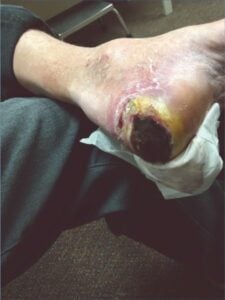 Patient presented with Patient presented withosteomyelitis and underlying purulent tissue |
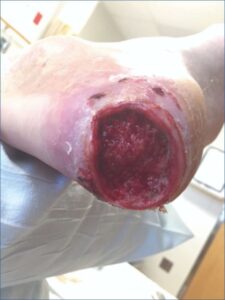 Post-Debridement + NPWT Post-Debridement + NPWTinitiated |
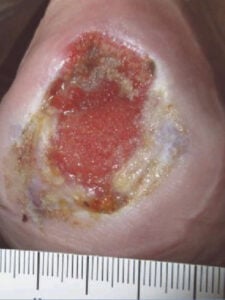 Week 8: First EPIFIX application after insurance coverage was received Week 8: First EPIFIX application after insurance coverage was received |
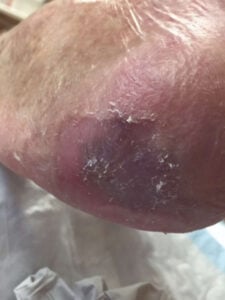 Week 15: Wound closed, s/p 4 total EPIFIX applications Week 15: Wound closed, s/p 4 total EPIFIX applications |
Case Example 2
Exposed Achilles Tendon: EPIFIX
84-year old male with multiple comorbidities and exposed Achilles tendon. Patient had 20 years of steroid use and prior closure attempts including biologic treatments which had failed one year prior to presentation. One week after initial debridement, no granulation tissue was present, so EPIFIX was applied.
Comorbidities: PVD and pyoderma
Risk Factor: 20 years of steroid use and one year history of failed prior closure attempts
Goal: Bridge to STSG
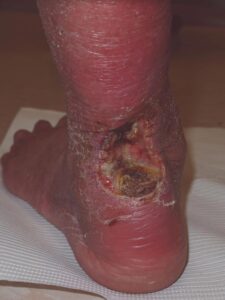 Initial examination Initial examination |
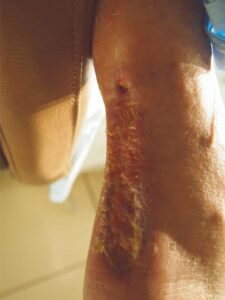 Week 6: s/p one EPIFIX Week 6: s/p one EPIFIXapplication. Healthy granulation prep for STSG |
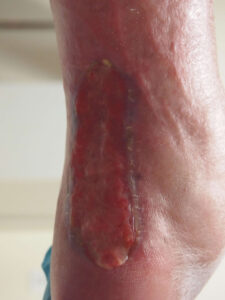 Week 16: STSG mostly Week 16: STSG mostlyclosed except small area above grafted area |
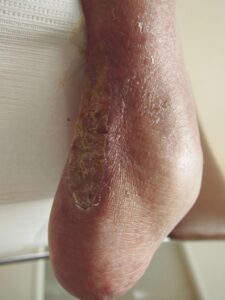 Week 22: Wound closed Week 22: Wound closedand stable |
Case Example 3
Venous Leg Ulcer (VLU): EPIFIX + NPWT
EPIFIX + NPWT in a comorbid patient with 3-year old chronic VLU that failed multiple treatments (e.g., HBOT, skin substitutes, biologics and two skin graft attempts with NPWT).
Comorbidities: DM, CVI and HTN
Goal: Secondary Intention Wound Closure
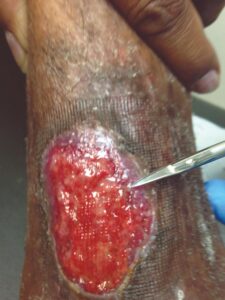 Week 1: s/p debridement Week 1: s/p debridementEPIFIX + NPWT |
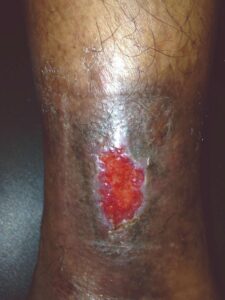 Week 2 Week 2 |
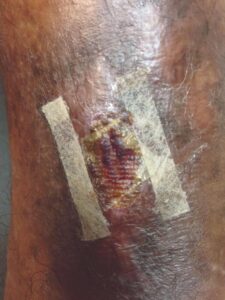 Week 3 Week 3 |
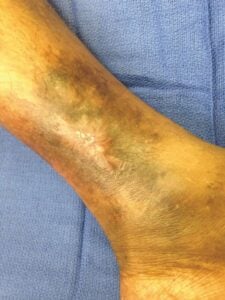 Day 35: Wound stable at 3, Day 35: Wound stable at 3,6, and 12 Month follow-ups |
Clinical Scenarios
| EPIFIX | EPICORD | EPIEFFECT | |
| Shallow wounds | ✓ | ✓ | ✓ |
| Defect below level of skin | ✓ | ✓ | ✓ |
| Deep/ tunneling, irregular shaped | ✓ | ✓ | ✓ |
| Fixation desired* | ✓ | ✓ | |
| Thickness desired | ✓ | ✓ | |
| Perforated/ Fenestrated | ✓ | ✓ | |
| Broad size assortment | ✓ | ✓ |
*Not intended for use as a load bearing tissue.
Which Product is Right for Me?
EPIEFFECT, EPIFIX, & EPICORD: A PART OF YOUR BEST-PRACTICES TREATMENT PROTOCOL
MIMEDX offers a portfolio of advanced placental-based allografts in the hospital outpatient department or physician office setting. EPIEFFECT, EPIFIX, and EPICORD are available in a variety of sizes to meet the needs of physicians and reduce graft wastage.
References
1. Koob TJ, Lim JJ, Massee M, Zabek N, Denozière G. Properties of dehydrated human amnion/chorion composite grafts: Implications for wound repair and soft tissue regeneration. J Biomed Mater Res B Appl Biomater. 2014;102(6):1353-1362.
2. Lei J, Priddy LB, Lim JJ, Massee M, Koob TJ. Identification of Extracellular Matrix Components and Biological Factors in Micronized Dehydrated Human Amnion/Chorion Membrane. Adv Wound Care (New Rochelle). 2017;6(2):43-53.
3. MIMEDX Internal Report. MM-RD-00086, Proteome Characterization of PURION Processed Dehydrated Human Amnion Chorion Membrane (dHACM) and PURION PLUS Processed Dehydrated Human Umbilical Cord (dHUC) Allografts.
4. Bullard JD, Lei J, Lim JJ, Massee M, Fallon AM, Koob TJ. Evaluation of dehydrated human umbilical cord biological properties for wound care and soft tissue healing. J Biomed Mater Res B Appl Biomater. 2019;107(4):1035-1046.
5. Zelen CM, Serena TE, Denoziere G, Fetterolf DE. A prospective randomised comparative parallel study of amniotic membrane wound graft in the management of diabetic foot ulcers. Int Wound J. 2013;10(5):502-507.
6. Zelen CM. An evaluation of dehydrated human amniotic membrane allografts in patients with DFUs. J Wound Care. 2013;22(7):347-351.
7. Zelen CM, Serena TE, Fetterolf DE. Dehydrated human amnion/chorion membrane allografts in patients with chronic diabetic foot ulcers: A long-term follow-up study. Wound Medicine. 2014;4:1-4.
8. Zelen CM, Serena TE, Snyder RJ. A prospective, randomised comparative study of weekly versus biweekly application of dehydrated human amnion/chorion membrane allograft in the management of diabetic foot ulcers. Int Wound J. 2014;11(2):122-128.
9. Zelen CM, Gould L, Serena TE, Carter MJ, Keller J, Li WW. A prospective, randomised, controlled, multi-centre comparative effectiveness study of healing using dehydrated human amnion/chorion membrane allograft, bioengineered skin substitute or standard of care for treatment of chronic lower extremity diabetic ulcers. Int Wound J. 2015;12(6):724 -732.
10. Zelen CM, Serena TE, Gould L, et al. Treatment of chronic diabetic lower extremity ulcers with advanced therapies: a prospective, randomised, controlled, multi-centre comparative study examining clinical efficacy and cost. Int Wound J. 2016;13(2):272-282.
11. Tettelbach W,Cazzell S, Reyzelman AM, Sigal F, Caporusso JM, Agnew PS. A confirmatory study on the efficacy of dehydrated human amnion/chorion membrane dHACM allograft in the management of diabetic foot ulcers: A prospective, multicentre, randomised, controlled study of 110 patients from 14 wound clinics. Int Wound J. 2019;16(1):19 -29.
12. Serena TE, Carter MJ, Le LT, Sabo MJ, DiMarco DT; EpiFix VLU Study Group. A multicenter, randomized, controlled clinical trial evaluating the use of dehydrated human amnion/chorion membrane allografts and multilayer compression therapy vs. multilayer compression therapy alone in the treatment of venous leg ulcers. Wound Repair Regen. 2014;22(6):688-693.
13. Bianchi C, Cazzell S, Vayser D, et al. A multicentre randomised controlled trial evaluating the efficacy of dehydrated human amnion/chorion membrane (EPIFIX® ) allograft for the treatment of venous leg ulcers. Int Wound J. 2018;15(1):114-122.
14. Bianchi C, Tettelbach W, Istwan N, et al. Variations in study outcomes relative to intention-to-treat and per-protocol data analysis techniques in the evaluation of efficacy for treatment of venous leg ulcers with dehydrated human amnion/chorion membrane allograft. Int Wound J. 2019;16(3):761-767.
15. Tettelbach W, Cazzell S, Sigal F, et al. A multicentre prospective randomised controlled comparative parallel study of dehydrated human umbilical cord (EpiCord) allograft for the treatment of diabetic foot ulcers. Int Wound J. 2019;16(1):122-130.
